The Gas Laws and Physics of Diving
The Gas Laws and Physics of Diving

So now that we’ve got the diving introduction out of the way, let’s jump right into what we feel is a major issue. When it comes to SCUBA Diving, it is important to know your Gas Laws. In Dive Physics, during the Second Phase of BUD/s, they teach you that these laws are very important to a diver. A diver must be aware of the physics affecting him underwater. Without the knowledge of what happens to the human body underwater, circumstances could easily become disastrous.
In this article, we’re going to cover six important diving laws and principles that every diver needs to be aware of. We urge you to study this article and develop a full understanding of how these laws work and to draw your own opinions about why they’re important. The definitions are taken from documents provided in BUD/s training, and we can’t think of anyone better to talk to about the water than the SEALs.
Boyle’s Law
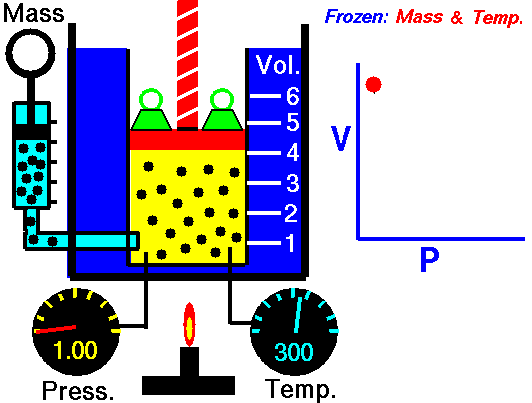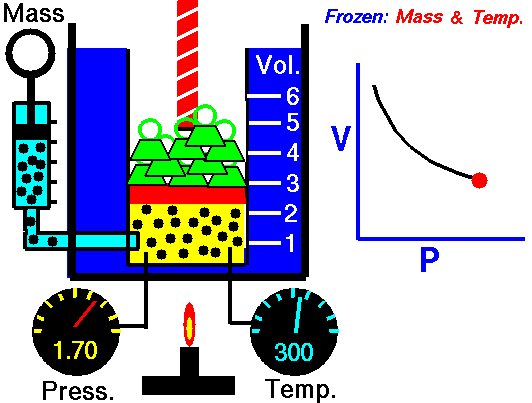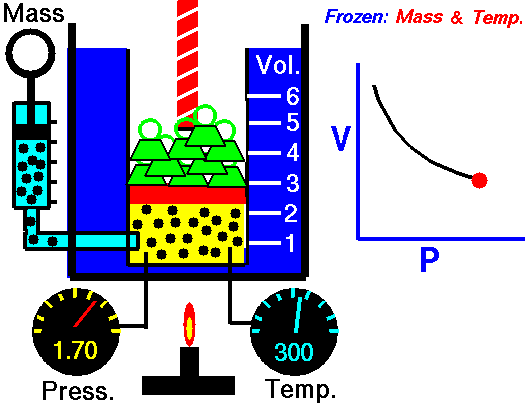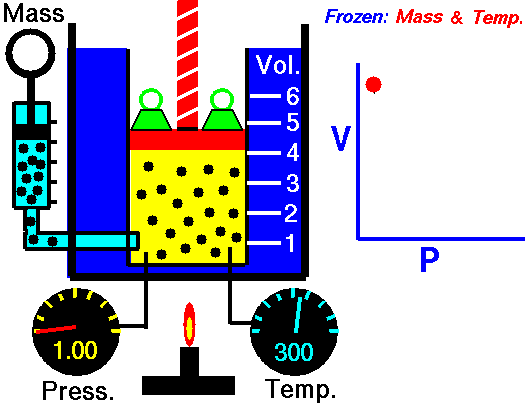
Boyle’s Law states that “given a constant temperature, the volume of a gas varies inversely as the absolute pressure.”
This law can be interpreted any way you like, but we should stress that this law is dependent on the absolute pressure. That means that as the pressure increases, the volume decreases. It would be silly to think that you could decrease the absolute pressure simply by increasing the volume of a gas, so it is fair to say that as pressure decreases, the volume will increase.
To illustrate this law, we take a bottle full of air down to a depth of about 100ft (30m), 3 ATM (atmospheres gauge) or 4 ATA (atmospheres absolute). As the bottle descends, the absolute pressure around the bottle forces the gas (Air) inside the bottle to compress therefore allowing for less volume. This happens because as you go deeper into water, the pressure increases at a rate of one ATM every 33ft (10m). This is the pressure that decreases the volume of gas inside your bottle.
Lets say we take that bottle up into the air 36,000 ft. (10,970m), cruising altitude for a standard commercial jet liner. The air in the atmosphere actually has a lower absolute pressure than we do here on the surface. You may have heard a little rumor about how space is a vacuum, well this is that transitional point from our perfect living conditions. Now, using Boyle’s Law we can conclude that the bottle will actually grow as the absolute pressure around it decreases. The difference here is that the pressure changes are not as drastic in the atmosphere of planet Earth as they are in the water.
Charles’s Law / Gay-Lussac’s Law
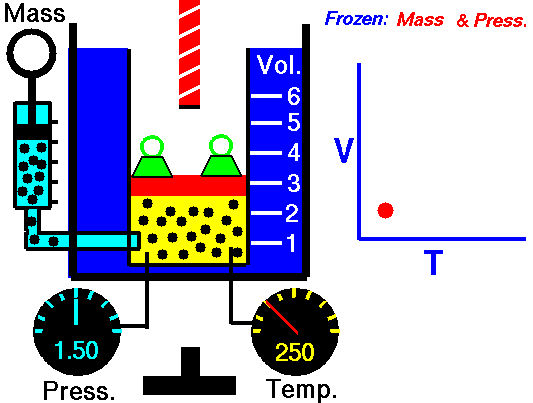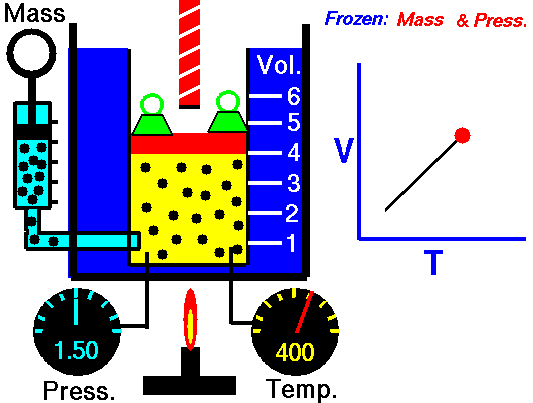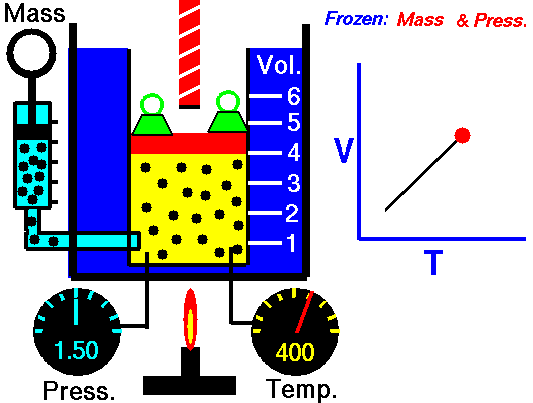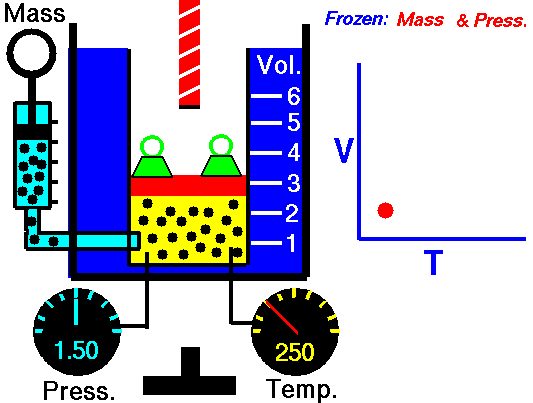
Charle’s law states that given a constant pressure, the volume of a gas varies directly as the absolute temperature. This law is sometimes referred to as Gay-Lussac’s Law.
This is probably one of the simpler laws. The same principles are in play most often in your daily life. Most of you know that as metal gets hot or cold, it expands and contracts (more notably than other things like plastic). Well gases do the same thing.
What Charles’s Law says is that if you take a volume of air, and keep it at a constant pressure, you can increase your volume simply by heating it up. You could also effectively reduce the volume by putting it in a deep freeze, or setting it outside if you live up north. So to illustrate this law, we use a SCUBA tank.
When a SCUBA cylinder is filled, the friction of the air entering the cylinder heats things up from the inside out. This makes the surface of the cylinder warm to the touch. Just about all of your shops will overfill cylinders by about 10%, which is the maximum allowed by the DOT. There is a reason for this.
Every cylinder model has a different “working pressure,” which means that they can handle being filled to lets say 3000 psi (206 bar), plus 10% of that pressure. So then we have a cylinder that gets filled to 3300 psi (227 bar). This is not a safe working pressure for the cylinder (theoretically), so why do we fill a cylinder to more than its safe working pressure? They are overfilled because of Charle’s Law. Friction heats the cylinder and that cylinder will eventually cool off, thus, decreasing the volume of air inside. So to counter this effect, dive shops put more air in the cylinder than would be necessary for a typical dive.
Dalton’s Law
 Dalton’s Law states that the total pressure exerted by a mixture of gases is the sum of the pressures exerted by each of the gases.
Dalton’s Law states that the total pressure exerted by a mixture of gases is the sum of the pressures exerted by each of the gases.
This is pretty straight forward. It becomes most useful for anyone qualified to fill a scuba cylinder. Lets say your target working pressure is 3000 psi (206 bar) for a typical SCUBA cylinder fill. Normally, you would take the air from the room you are standing in, compress it down, and pump it into a cylinder. Some people prefer the use of different breathing gases. For simplicity’s sake, we’ll use the breathing gas “NITROX.” There will be an article on the different gases, so don’t panic if you are a little confused by this. The air we breath is an average 21% Oxygen 79% Nitrogen. Nitrox is a gas with an oxygen content of 22%-40%. The benefits of breathing oxygen rich gas are numerous, but we’ll cover that as well at a later date.
So, to reach a higher oxygen content, there are formulas that need to be followed. Many factors go into consideration, such as working pressure, cylinder volume, percent of O2 desired, etc. The basic formula says this: start with Xpsi of 100% O2, and then fill to 3000 psi (206 bar) with air. This gives the desired percentage of oxygen in your nitrox gas. So we have a hypothetical 400 psi (27 bar) of pure oxygen, and we will top off the cylinder with 2600 psi (179 bar) with air, leaving 3000 psi (206 bar) of pressure in the cylinder. 400 + 2600= 3000psi (27+179=206 bar). The total pressure is equal to the sum of the pressure of each part of the mixture.
Henry’s Law
Henry’s Law states that the amount of gas that will dissolve in a liquid is directly proportional to the partial pressure of that gas.
Here is a law that goes much deeper than mixing gases and heating SCUBA cylinders. This is the mother of dive laws in our opinion, but we believe others would agree. This law does not reference any of what has been discussed previously. We could think of ways to get gases to dissolve into fluids, but this law should be presented in the manner it was intended.
If you’ve taken a diving class, you know that nitrogen is your biggest opponent underwater. Nitrogen happens to be a gas, but we’re not so worried about the nitrogen absorbing into the water at higher pressures. Not hardly. You see, while you’re on the surface, nitrogen absorbs into your skin. This is not a real issue while you’re walking around, driving your cars, etc. Take that body down to 100 ft (30 m) of water and you change the circumstances dramatically.
During your time underwater, the pressure around your body forces nitrogen into your blood stream. This can (but not always) lead to a rather pleasant narcotic feeling at depth we refer to as “nitrogen narcosis.” Nitrogen has a narcotic effect on the brain which can be alleviated by ascending to a shallower depth until symptoms pass. The longer you stay, and the deeper you go, the more nitrogen will be forced into your blood stream. If you don’t get narcosis easily, then you will most likely not notice the build up of excess nitrogen in your blood. We know this gas is present because of very extensive research conducted by Navies around the world, more notably the US Navy which releases nearly a thousand page manual on diving to certain jobs.
The nitrogen at depth is not the issue, but rather when you complete your dive and begin your ascent to the surface. As you ascend, the nitrogen gas that has dissolved into your blood stream will begin to form bubbles as the gas expands. If you were to acquire an excess of nitrogen and immediately get out of the water, within about 15 minutes you would begin showing symptoms of DCS (decompression sickness) which can be fatal if not treated. This sickness has been dubbed “the bends,” or “Cassien’s disease” by divers in the past. This build up of nitrogen is removed from the body by ascending to a shallower depth and allowing the nitrogen to pass through your blood stream, and into your lungs. Once in your lungs, the nitrogen returns to its undissolved, gas state and passes from your body as you exhale. The depth and amount of time required for “off-gassing” is directly related to the amount of time, and depth of your dive.
Dive Tables
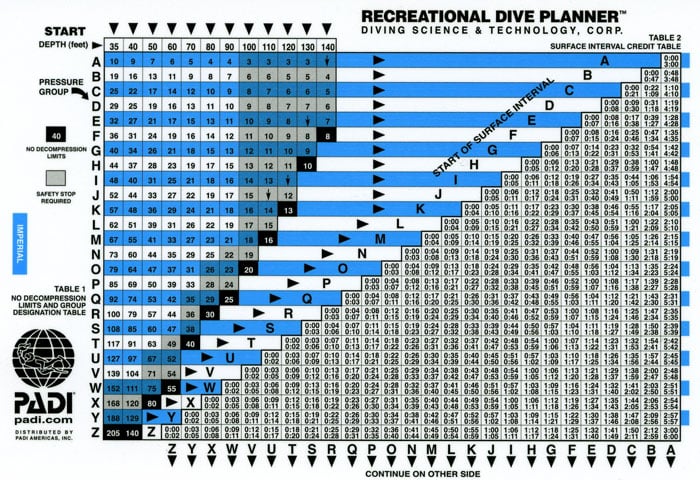
To prevent death or nerve damage, the US Navy released what is a called a “dive table.” If you are a certified diver, you will have been required to dive from a table to acquire your certification. For those who may be unfamiliar, a standard air diving table basically lets you know how much nitrogen you will build up at any depth for any amount of time. This basic table is a “no-decompression dive table,” which means that at any depth (to 130 ft or 40m) for any time allotted on the table, you will not be required to stop and off-gas nitrogen since you have not acquired any excess in your bloodstream. Tables can be quite confusing if you have never used one before so I will break it down for you.
In a nutshell, you select the depth at which you plan on diving. Always use the deepest point of the dive, even if it’s only for a minute unless you are using a planning wheel which will be covered at a later date. The depths are typically listed in both feet and meters so everyone can safely understand them. Next, move down the row to the corresponding number of minutes you plan on staying. You may notice that the deeper you plan on going, the less time you have available for the actual dive. The numbers represent the maximum amount of dive time allowed per dopplar no-decompression diving limits. Any longer, and you are in a decompression dive, which is not necessarily a dangerous situation, but you must be prepared for decompression stops. Some dive tables have mild decompression diving information on them used generally for emergency situations.
The tables help you understand how much nitrogen has been absorbed into your bloodstream, and what precautions must be taken to prevent serious illness that may prevent you from diving in the future. Going beyond the dopplar limits without proper training could prove to be fatal.
Archimedes Principle
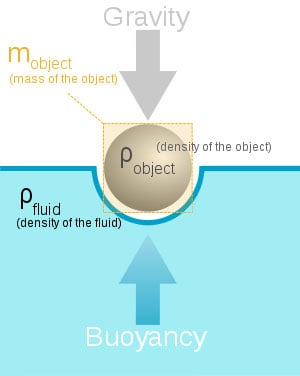 Archimedes Principle states that and object wholly or partially immersed in a liquid is buoyed up by the force equal to the weight of the liquid displaced.
Archimedes Principle states that and object wholly or partially immersed in a liquid is buoyed up by the force equal to the weight of the liquid displaced.
This one is very simple to understand. Lets say we fill a cup with water until it’s about to spill over the edge. Then we take a rock, and drop it in that cup, the water would begin to flow over onto the table. Now to find the value referenced by Archimedes Principle, we would simple take the water that spilled, and weigh it. Lets say for the sake of argument the displacement of the water weighs eight grams. That means, according to the principle in discussion, that the rock is being pushed back up with a force of 8 grams.
This law is similar to Newton’s law of relative motion which states: for every action, there is an equal and opposite reaction. This is basically the same theory, we are just talking about objects being immersed in fluid, rather than friction and motion.
We use this law for many different things while in the water. It is mostly used to calculate the specific buoyancy of an object. This will be important for anyone that spends time underwater. For instance, if your Buoyancy Compensation Device does not have the lift capacity to bring up your steel SCUBA cylinders, you will surely be stuck on the bottom. We can set values to plan these things because of Archimedes Principle.
This law doesn’t only apply to the underwater community. Naval Architects will use this principal to calculate many different requirements of a ship’s hull. An aircraft carrier is made mostly out of steel, but because of the way she’s designed, she floats. If you were to place any discrepancy in the hull of a carrier below the water line, the consequences could be dire. The same goes for the laws of density. If you have two pieces of bread, and you throw one in the water like it is, then it will float, but if you crush the other into a ball and toss it, it will surely sink. This is the issue with ships as well, if we use Archimedes Principle along with other amazingly simple laws, we have enormous metal structures floating and moving under their own power.
Notes
We’d like to thank Derek Gill for his help in getting these gas laws rounded up into an article for ITS, and appreciate all his help. Please let us know if you have any further questions regarding any of these gas laws, and be sure to throw up a comment. We look forward to bringing you more information in the future regarding diving, and would like to hear what you’re interested in!
Disclaimer
ITS Tactical cannot be held responsible for any attempts at any form of diving without first seeking professional training and advice. The following article is not intended as a replacement for proper training and equipment used in any water sport activity. Diving is inherently dangerous, and introduces a unique set of risks not typically present in everyday life. We urge you to seek proper instruction from a qualified, and certified agency before attempting any sport requiring a life support system, namely: SCUBA. It is likewise very important that you contact your health care provider before attempting any training classes to ensure you are in good physical condition. Those with pre-existing medical conditions may be at a higher risk for certain complications that may become present while SCUBA Diving.











Discussion